Currently, vanadium redox flow batteries (RFBs) are the most recognized among flow batteries. However, other RFB technologies also deserve attention; for example, Zn-Br2, Fe-Cr, Fe-Cd, Zn-Fe, and Zn-Ni systems.
Vanadium redox flow batteries
Among all the RFBs technologies, vanadium systems are the most well-researched. The use of vanadium ions in different oxidation states as catholyte and anolyte helps to suppress crossover in the system.
| Negative electrode | V3+ + e– → V2+ |
| Positive electrode | VO2+ – e– + H2O → VO2+ + 2H+ |
Electrolytes based on concentrated acid solutions exhibit high conductivity that makes it possible to achieve battery Energy Efficiency of 80% at current densities of 100 mAꞏcm-2. However, vanadium RFBs exhibit relatively low voltage (1.4 V) and energy density (25 Wh L-1), as well as a limited operating temperature range (10 – 40°C).
Zink-bromine redox flow batteries
Apart from vanadium systems, commercialized technologies also include flow batteries based on Zn/Br2.
| Negative electrode | Zn2+ + 2e– → Zn0 |
| Positive electrode | 2Br– – 2e– → Br2 |
These batteries boast higher voltages (1.8 V) and a practical energy density of 60 Wh L-1 (theoretical energy density of 570 Wh L-1). The key advantage of this technology is the low price of active materials, making it possible to reduce electrolyte costs to <42 USD per kWh. The main challenges are: crossover (the system uses mixed electrolytes to suppress it), the possibility of the zinc dendrite formation, kinetics imbalance, and the formation of polybromide anions. The main strategies to address these issues involve electrode modification, the development of new ion exchange membranes, and modification of the electrochemical cell design.
Iron-Chromium Redox Flow Batteries
The Fe/Cr system-based flow batteries, conceived by NASA in the 1970s for deep space missions, utilize common and economical materials. The battery cell voltage reaches 1.18 V.
| Negative electrode | Cr3+ + e– → Cr2+ |
| Positive electrode | Fe2+ – e- → Fe3+ |
The technological challenge includes the slow kinetics of the Cr2+/Cr3+ electrochemical reaction, requiring a catalyst, and the crossover of active components. However, both vanadium and Fe/Cr system-based batteries represent high electrochemical stability and efficiency, with minimal capacity loss (0.3% and 1.2% per cycle) and energy efficiency (80.3% and 78.4% at 120 mAꞏcm−2) for vanadium and Fe/Cr batteries, respectively.
All-Iron Redox Flow Batteries
The first all-iron redox flow batteries were developed in 1981, reaching a voltage of 1.21 V and a theoretical volumetric specific capacity of 76 W∙L-1. Unfortunately, low current efficiency values and insufficient stability do not allow the widespread implementation of this technology. Exploring the use of iron complexes instead of simple inorganic salts is a potential development direction.
| Negative electrode | Fe2+ + 2e– → Fe0 |
| Positive electrode | 2Fe2+ – 2e– → 2Fe3+ |
Polysulfide/Bromide System Flow Batteries
This type of flow battery utilizes sodium polysulfide and NaBr as anolyte and catholyte, respectively.
| Negative electrode | Sn2- + 2e– → 2Sn2- |
| Positive electrode | 3Br– – 2e– → Br3– |
The main advantage lies in the availability and low cost of redox-active materials, while the primary disadvantage is the cross-charging of electrolytes, necessitating an enhanced control system for charging and discharging processes.
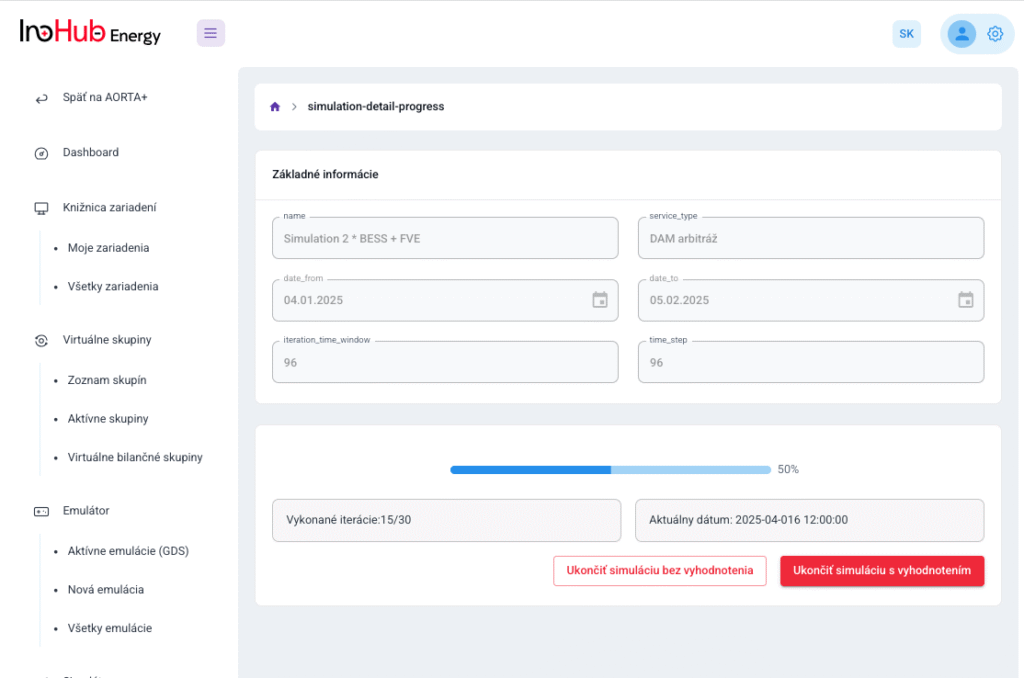
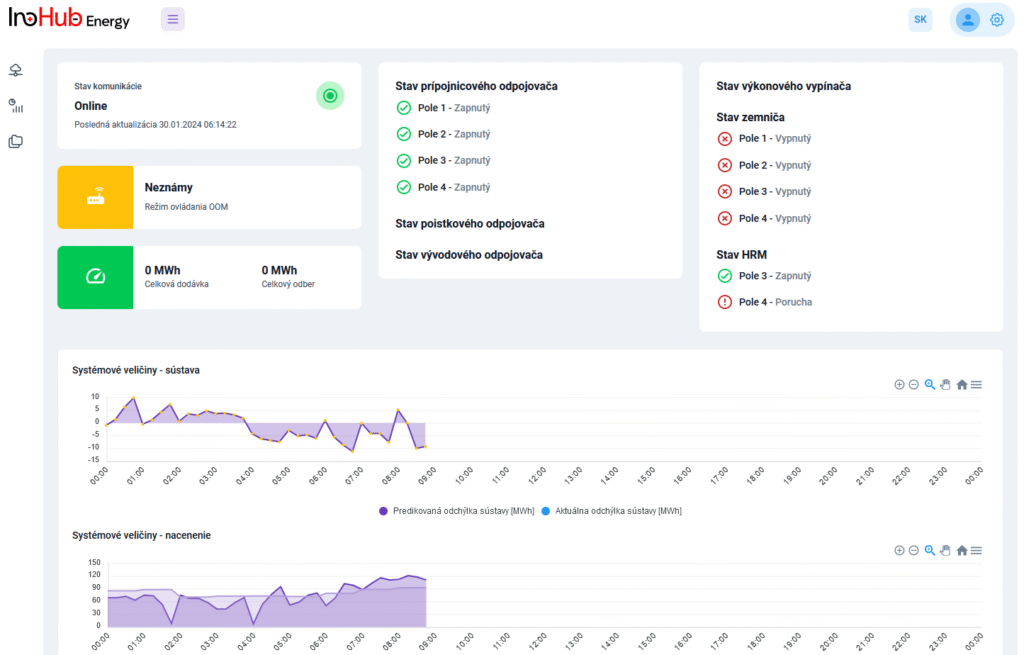
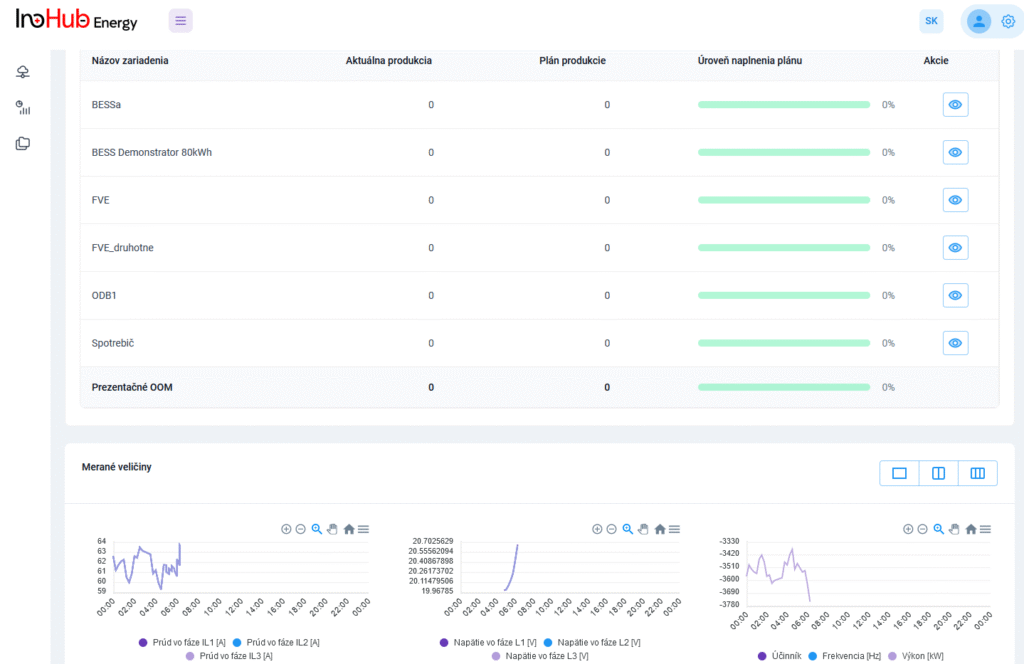
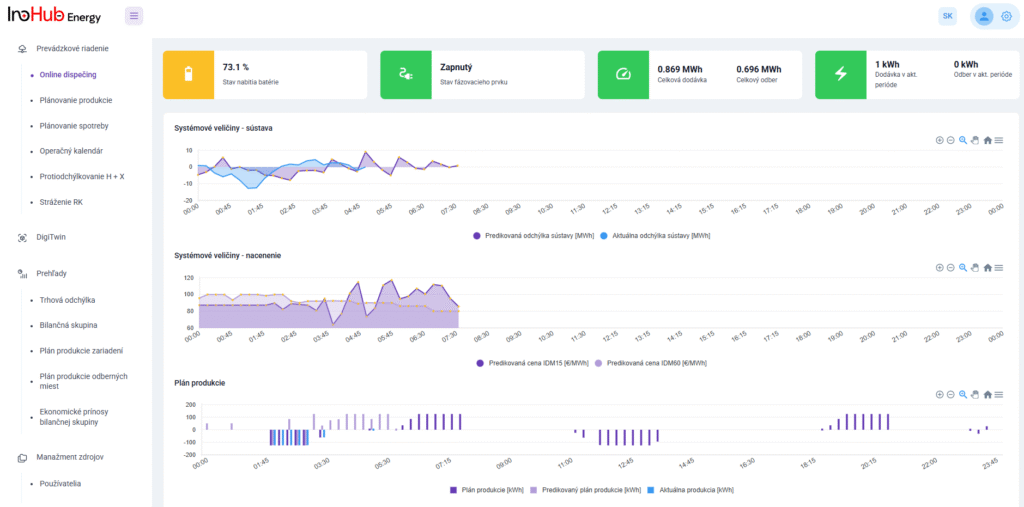
INO-HUB Energy R&D team in collaboration with UPJS University is planning to start research activities on vanadium RFBs, including the optimization of vanadium electrolyte composition and cell geometry.
ACKNOWLEDGEMENT:
This work was supported by the project: IPCEI_IE_FLOW_BESS_012021