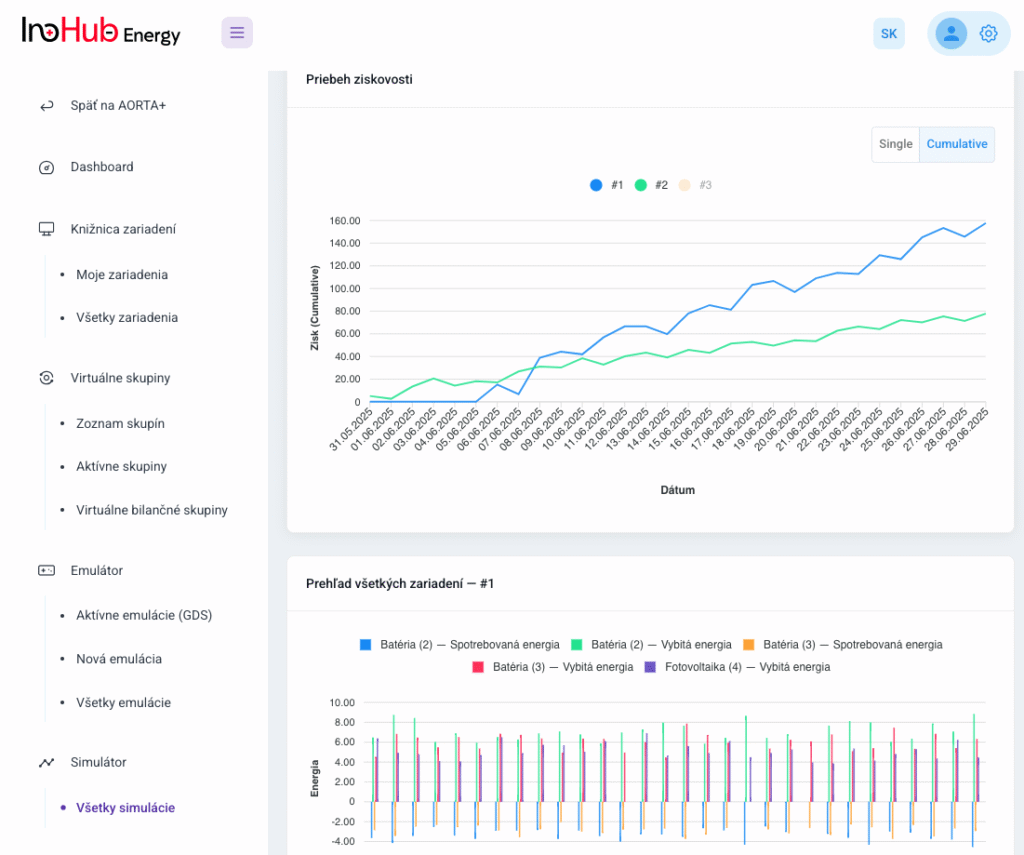
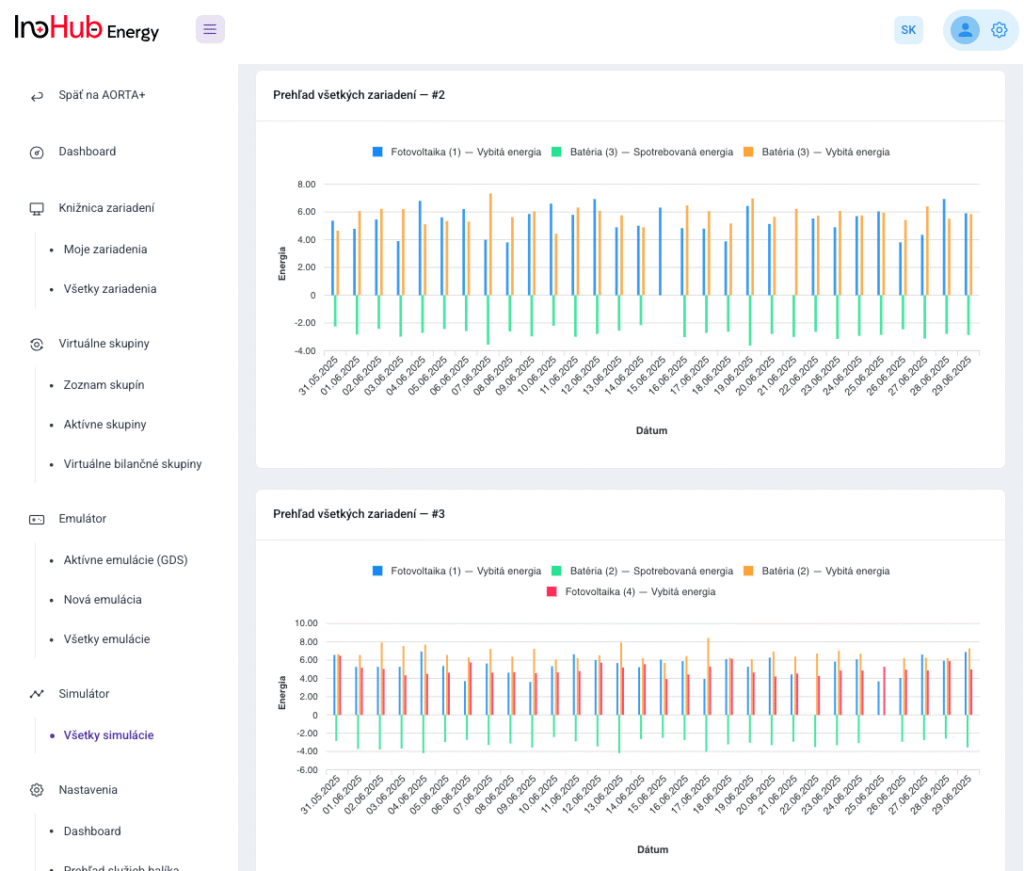
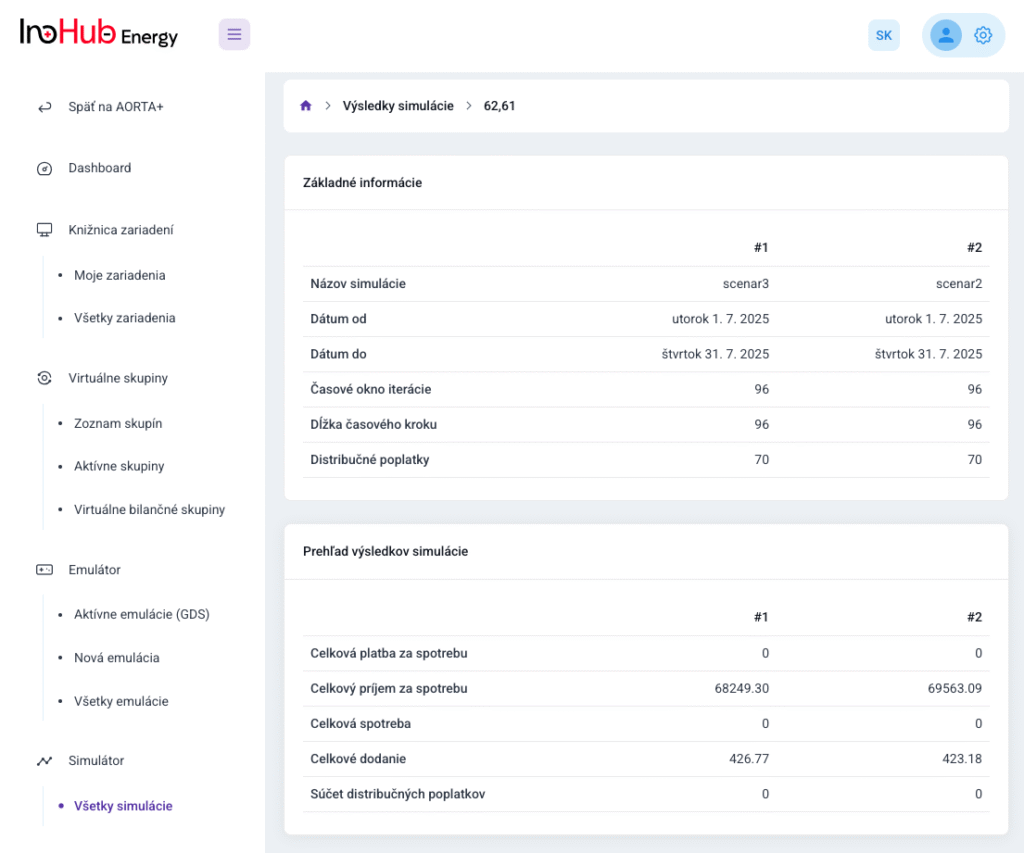
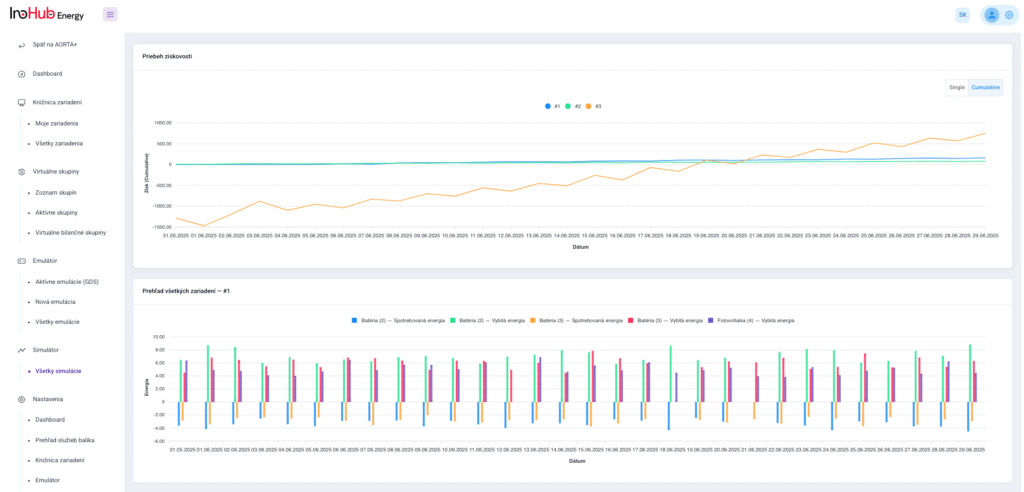
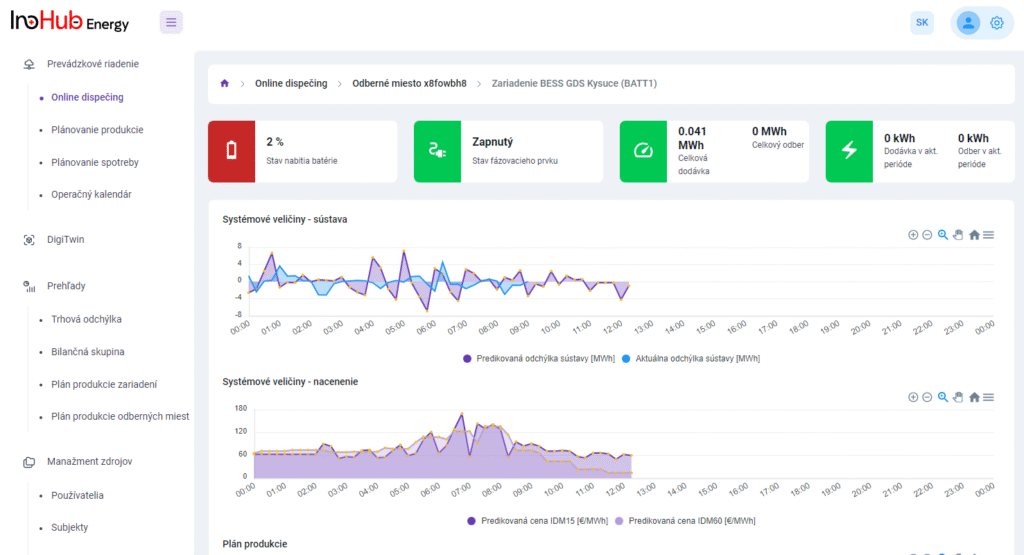
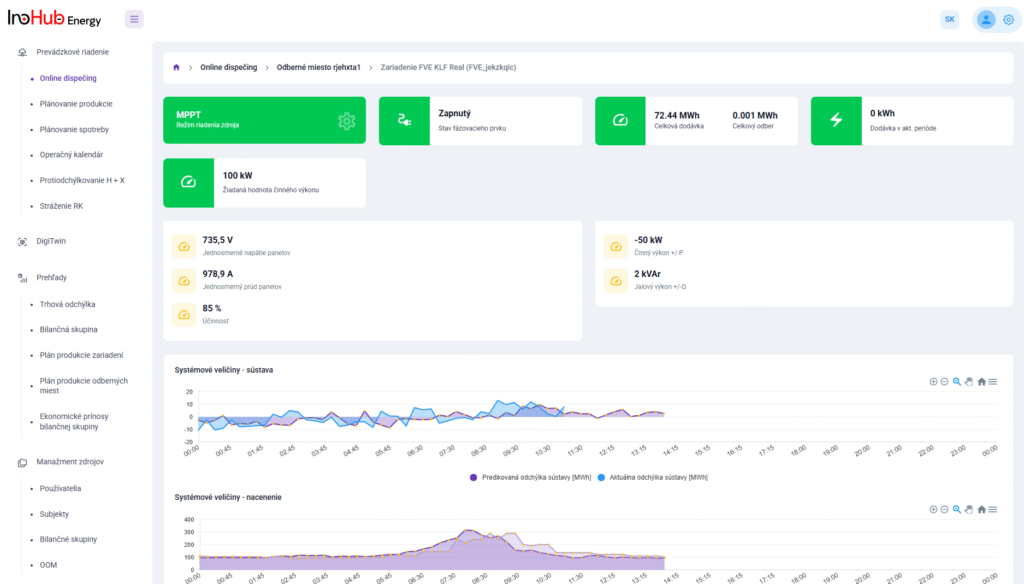
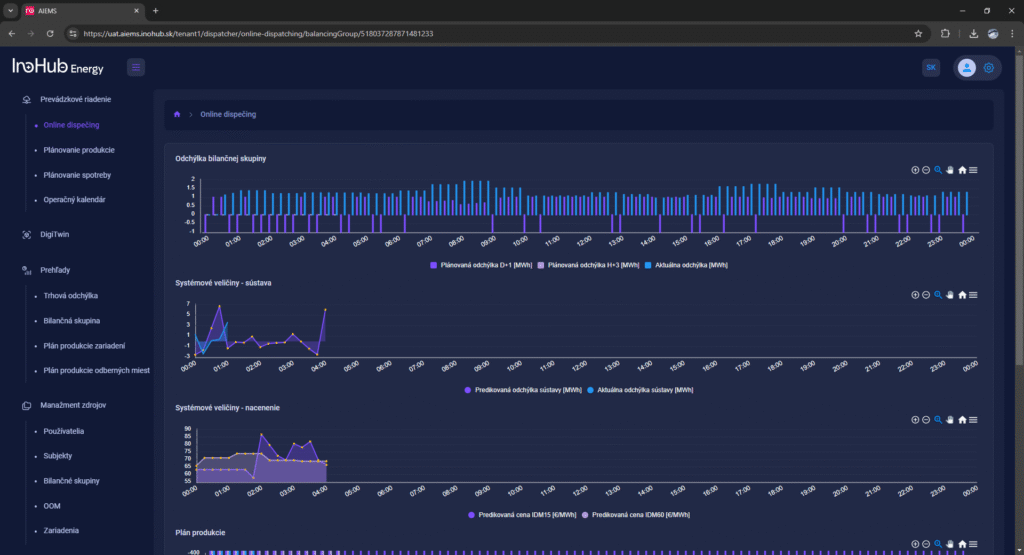
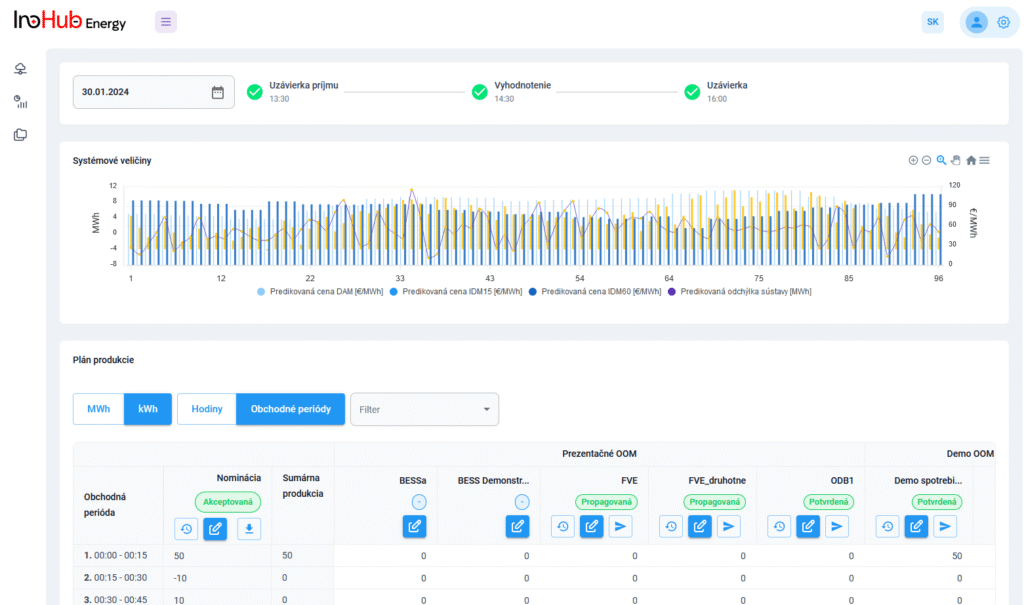
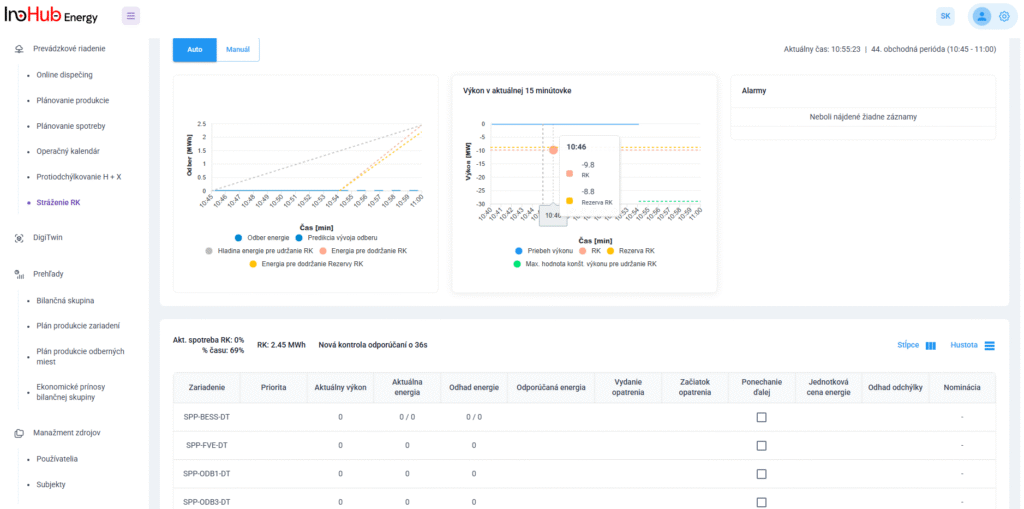
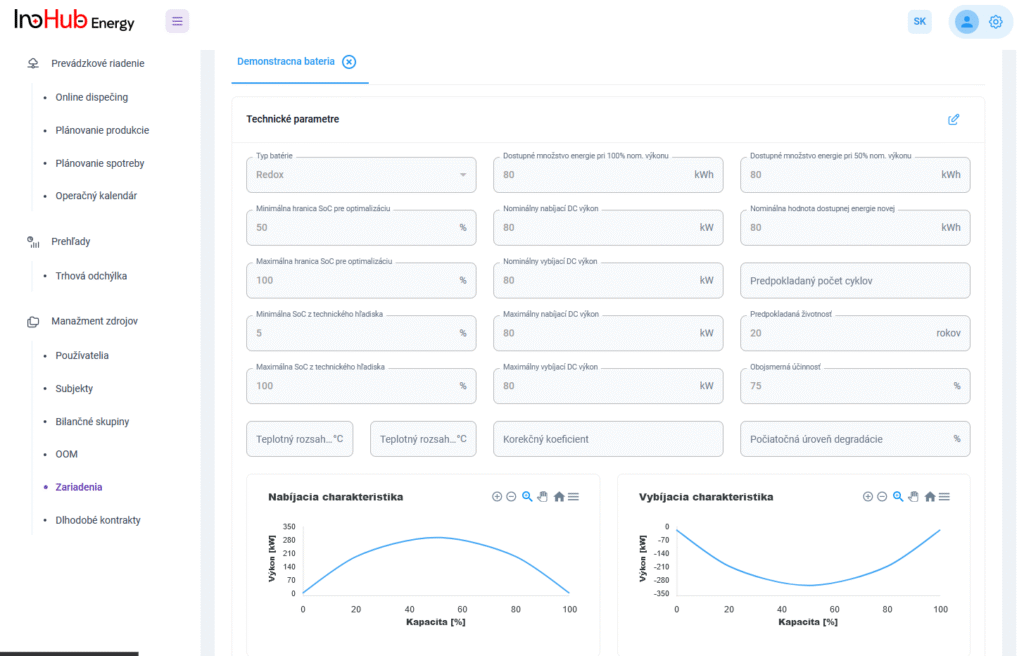
V INO-HUB Energy sme odhodlaní vyvíjať inovatívne alternatívy elektrolytov na základe redoxne aktívnych skupín organických zlúčenín rozpustných vo vode. Organická chémia otvára potenciál na vytváranie elektrolytov z prvkov hojne rozšírených na zemi, čím podporuje udržateľné a ekologické riešenia.
Jednou z kľúčových výhod týchto organických zlúčenín je ich vysoká prispôsobiteľnosť. Ich elektrochemické vlastnosti – ako je redoxný potenciál, rozpustnosť a životnosť cyklu, možno optimalizovať cielenými chemickými modifikáciami. Navyše vodné organické redoxné prietokové batérie (Aqueous Organic RFB) vynikajú svojou schopnosťou pracovať v širšom teplotnom rozsahu, vďaka čomu sú vhodné pre rôzne podnebné pásma a podmienky.
Napriek týmto výhodám zostávajú aj výzvy – ako zlepšiť stabilitu organických zlúčenín, znížiť náklady na syntézu a zvýšiť rozpustnosť. V INO-HUB riešime tieto výzvy skúmaním nových zlúčenín s rôznymi redoxne aktívnymi organickými molekulami.
Chinóny
Táto skupina je inšpirovaná biologickými systémami transportu elektrónov (napr. fotosyntézou). Patria sem hydrochinóny, antrachinóny a benzochinóny. Chinóny ponúkajú vysokú reverzibilitu, rýchlu kinetiku a dvojelektrónové redoxné reakcie. Zostávajú stabilné v kyslých aj zásaditých podmienkach. Napriek tomu potenciál vedľajších reakcií a ich obmedzená rozpustnosť sú naďalej výzvou.
Viologény
Viologény sa vyznačujú vysokou rozpustnosťou a rýchlou kinetikou. Taktiež vlastnosti týchto zlúčenín je možné upravovať. Obmedzená stabilita prenosu druhého elektrónu však komplikuje ich širšiu realizáciu.
Fenazíny
Tieto molekuly poskytujú dva využiteľné elektróny a vysoký redoxný potenciál, preto sú vhodné pre RFB s vysokou energetickou hustotou. Sú stabilné v zásaditých podmienkach, ale majú tendenciu vykazovať vedľajšie reakcie počas dlhšieho cyklu.
Komplexy železa
Tieto organokovové katolyty ponúkajú extrémne rýchlu kinetiku a vysokú reverzibilitu. Hlavnou nevýhodou je ich obmedzená rozpustnosť vo vode.
Deriváty Tempo
Ako nitroxylové radikály sa deriváty TEMPO používajú ako katolyty, kvôli ich vysokému redoxnému potenciálu a rozpustnosti vo vode. Problémom naďalej zostáva ich obmedzená stabilita vo vodných roztokoch.
Ďalšie skupiny
Malé skupiny ako napríklad flavíny a azobenzény si tiež získavajú pozornosť výskumníkov vďaka svojim jedinečným redoxným vlastnostiam a potenciálnym aplikáciám.
POĎAKOVANIE:
Táto práca bola podporená projektom: IPCEI_IE_FLOW_BESS_012021_2. fáza