Vanadium redox flow batteries have a unique chemistry compared to other redox flow battery systems because the operation starts with the same electrolyte at both catholyte and anolyte sides. This is possible due to the chemical composition of the starting electrolyte, which consists of two vanadium salts: VOSO₄ and V₂(SO₄)₃. These represent vanadium in two oxidation states—vanadium (+3) and vanadium (+4)—mixed together in approximately equal proportions. Thus, before the first charging of the battery, a pre-charging step is necessary. The standard vanadium electrolyte typically has a concentration of 1.6M vanadium; however, solutions with concentrations of 1.7 M, 1.8 M, or even 2.0 M are also available. Higher concentration solutions require more precise temperature control due to the increased likelihood of V(+5) precipitation on the positive electrode.
The main impurities that can affect electrolyte performance are:
- Copper and Nickel: These metals could precipitate on the negative electrode and accelerate hydrogen evolution.
- Aluminium: Aluminium can also precipitate on the electrodes and impact the kinetics of the negative electrolyte reaction.
- Silicon: The deposition of silicon oxide on the electrode can lead to reduced flow.
- Iron: Participation of the iron ions in the side electrochemical reaction reduces the battery capacity.
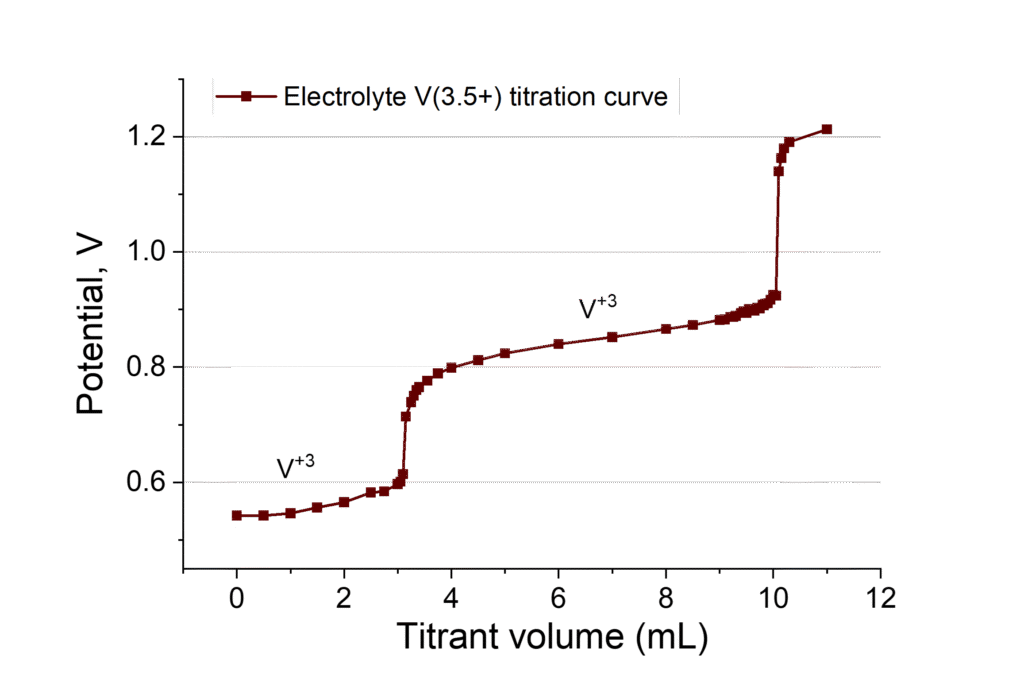
During battery cycling, side processes such as water transfer and vanadium ion crossover occur, resulting in changes to the electrolyte volume and imbalances in concentrations. The concentration and state of charge of the electrolyte on both sides can be monitored using UV-Vis measurements or by titrating the vanadium solution with an oxidizing agent, such as potassium permanganate.
At Inohub Energy, we place great emphasis on the quality of the electrolyte used in both our large systems and small cell testing, routinely controlling for concentration and impurities.
ACKNOWLEDGEMENT:
This work was supported by the project: IPCEI_IE_FLOW_BESS_012021_2. phase
References: